A Breakthrough in Treating Obstructive Sleep Apnea
Biological Tendon-like Tether Technology (BTTT) - An imminent solution to a global health crisis affecting 1 billion people worldwide.
The problem
1B
Adults affected worldwide
450M
Patients require intervention
€184B
Annual cost across the EU
30-60%
CPAP non-adherence rate
The BTTT Solution
How It Works
1
Surgical Implantation
A biological scaffold is implanted via a one-time surgical procedure
2
Natural Integration
The body grows a tendon-like structure, naturally integrating with the immune system
3
Permanent Solution
The result is a permanent anatomical correction that advances and anchors the tongue base
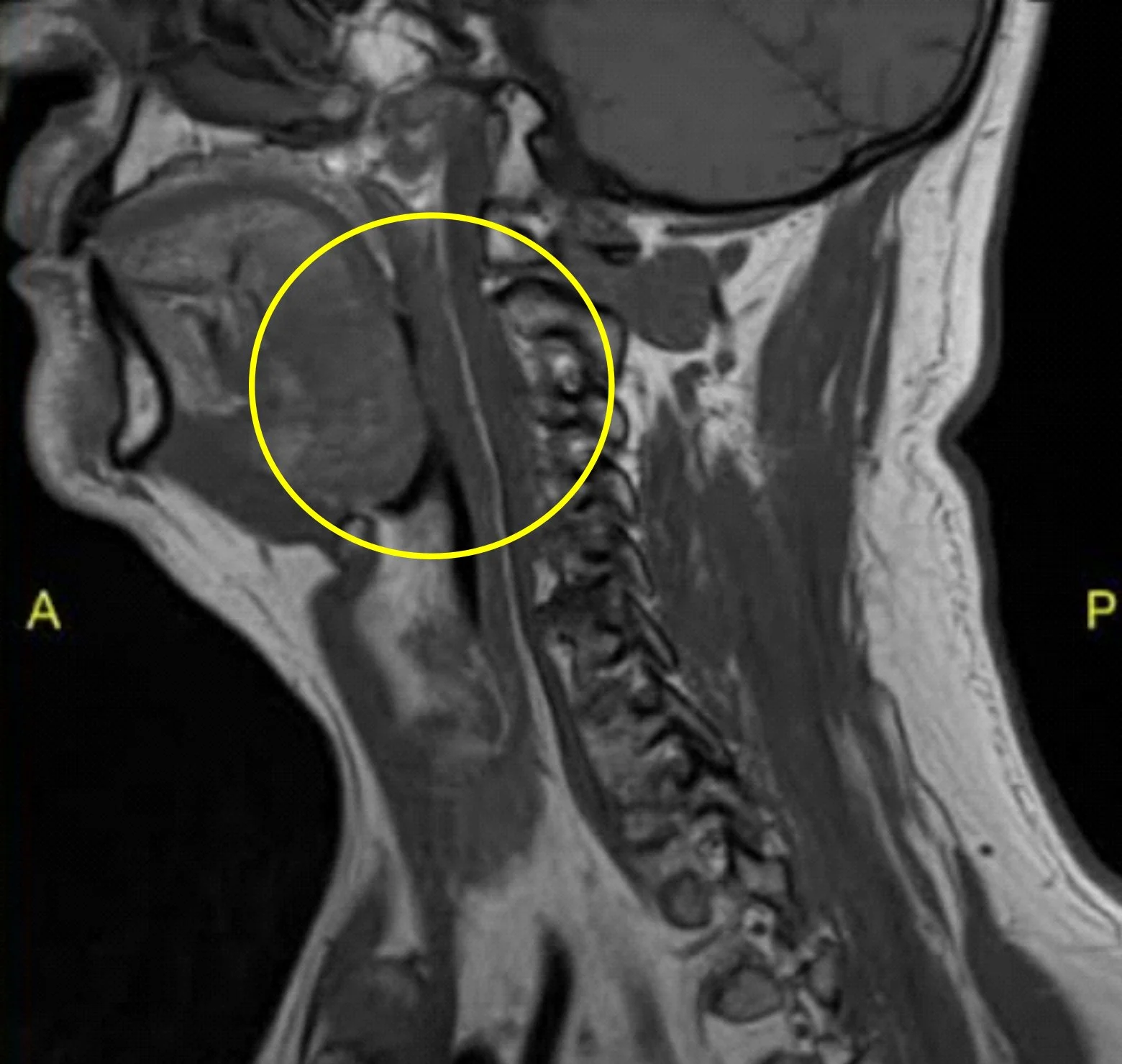
MRI SCAN BEFORE APNEACURE
Tongue Obstructed Airway
Chronic OSA
MRI SCAN AFTER APNEACURE
Clear Airway
No OSA
What Makes It Different
Passive System
No external intervention needed unlike CPAP
Addresses Root Cause
Prevents airway collapse during sleep
One-Time Treatment
No mechanical failure or degradation
Body heals itself
Completely dissolves after 8 months
Patents granted in USA, China, UK, European Union, France, Germany, Spain, and South Africa.
Clinical Validation
Ready for clinical trials.
Animal trials demonstrated successful tendon formation.
PCT patent application filed and granted in major global markets including USA and China.
Successful first human implantation marking a major milestone for the technology.
Clinical trial preparations are complete, and a robust safety and efficacy monitoring policy is in place.
Large-scale clinical validation to complete regulatory requirements.
Regulatory approval, scaled production, and commercial launch in key markets.
Development Milestones
Our Leadership Team
Prof. Mogamat Rushdi Hendricks
Founder & CEO / Chief Medical Officer
Lead surgeon and inventor with 30+ years experience. PhD in Plastic Reconstructive and Maxillofacial Surgery. Fellow of the Royal Society of South Africa. Pioneered BTTT technology.
Fakir Charles
Chief Executive Officer
Seasoned executive with 20+ years in management consulting, leveraging deep financial economics insight and entrepreneurial experience to drive strategic transformation and sustainable growth.
Lance Morel
Chief Operations Officer
Strategic operator with 30+ years leading, co-founding, and scaling ventures across finance, technology, and industrial sectors. Combines deep operational insight with transformative leadership to accelerate growth, efficiency, and market impact.
Antonie Botha
Chief Development Officer
Seasoned growth leader with 25+ years advancing disruptive technologies and innovative business models across global industries, delivering scalable development strategies and sustainable market impact.
FO
FO
Francois Oosthuizen
Project Manager
17+ years in innovation management, product development, and technology transfer. Skilled at aligning technical execution with strategic objectives to drive operational efficiency, stakeholder engagement, and sustainable growth.
MS
Dr. Marike Smit
Chief Technology Officer
A specialist in polymer and biomaterials science, she spearheaded the development of the BTTT scaffold technology, combining scientific rigor with translational vision to advance next-generation healthcare solutions.
Strategic Partners
University of Cape Town
R&D partner and shareholder. Provides research facilities, intellectual property support, and clinical expertise through the Division of Surgery.
Stocks-Charles AB
Commercial operations partner leading market entry, business development, internationalisation, and fundraising activities.
OSTA Biomedical
Regulatory partner providing quality management systems, regulatory compliance support, and certification processes.
About Us
Our Vision
To revolutionise the treatment of Obstructive Sleep Apnea by providing a permanent, biological solution that addresses the root cause of airway collapse, transforming the lives of millions of people worldwide and establishing a new standard of care in sleep medicine.
Our Mission
Innovate
Develop cutting-edge tissue engineering solutions that provide permanent anatomical correction for OSA patients
Deliver
Make BTTT technology accessible to patients worldwide through strategic partnerships and scalable production
Transform
Improve quality of life for OSA sufferers through a one-time surgical treatment with lasting results
Our Values
Innovation
Pioneering biological solutions through cutting-edge tissue engineering and research excellence
Patient-Centered
Prioritising patient well-being and quality of life in every decision we make
Excellence
Maintaining the highest standards in clinical validation, safety, and efficacy
Collaboration
Building strong partnerships with healthcare professionals, researchers, and institutions
Commercial Opportunity
$493.5M
Projected revenue in 5 years
705,000
Units/year addressable market
$1.2B
Projected gross profit in 8 years
10X
Investment opportunity within 8 years
Market Strategy
Distribution Model: Regional medical device distributors in key markets
Surgeon Training: Comprehensive certification program with regional training surgeons
Target: 2,350+ surgeons trained worldwide by year 5
Sales Channels: Certified surgical partners and established distribution networks
Production & Pricing
Current Unit Cost: Low cost with optimisation potential
Production: Modular, scalable on-demand manufacturing
Inventory: Low financial risk with distributor deposits
Quality: ISO 13485 compliant manufacturing processes
Investment Highlight
High-growth market with unmet medical need
Strong patent protection in major markets
Category-defining technology with scalable IP
Clear path to regulatory approval and commercialisation
Financial Projections
| Year | Units Sold | Revenue | Gross Profit |
|---|---|---|---|
| Year 1-2 | Clinical Trials | - | - |
| Year 3 | 35,250 | $61,6M | Initial Market Entry |
| Year 4 | 141,000 | $246,7M | Market Expansion |
| Year 5 | 282,000 | $493,5M | Scaling Phase |
| Year 8 | 705,000 | $1.2B | $1.1B |
Investment Proposition
Deal Structure
$11M
for market entry phase
Capital Raise
30%
Equity Equivalent
Projected Returns
10X in 8 years
based on conservative market estimates
Market Opportunity
$165B+
addressable market with limited competition in biological OSA solutions
Use of Funds
Clinical trials completion
Regulatory approvals
Scaling production
Surgeon training program
Commercial operations
For detailed investment materials, financial projections, and due diligence documentation, please contact:
Investor Registration
Join us in bringing a category-defining BioTech solution to market. Register your interest to learn more about our investment opportunity.